Acoustic Larviciding Technology
Acoustic Larvicide® is an innovative, chemical-free solution that uses the science of acoustics to target and eliminate mosquito larvae before they mature into biting adults. By harnessing sound waves at specific frequencies, we can disrupt the fragile respiratory system of developing larvae and collapse local mosquito populations sustainably.
Basics of Acoustics
Acoustics is the study of sound and how it moves through different mediums — air, water, or even solids. When objects vibrate, they create pressure waves that alternately compress and decompress molecules. These sound waves radiate outward in all directions, much like ripples spreading across the surface of a pond.
Sound is described by two main properties:
- Amplitude: The “loudness” or energy of the wave.
- Frequency: How many times a wave passes through a point per second, measured in hertz (Hz) or kilohertz (kHz).
When frequency matches the resonant frequency of an object, energy builds up dramatically — a principle called resonance. Classic examples include pushing a swing in rhythm or shattering a glass with sound. Resonance is also the foundation of Acoustic Larvicide®.
Acoustics and Mosquito Larvae
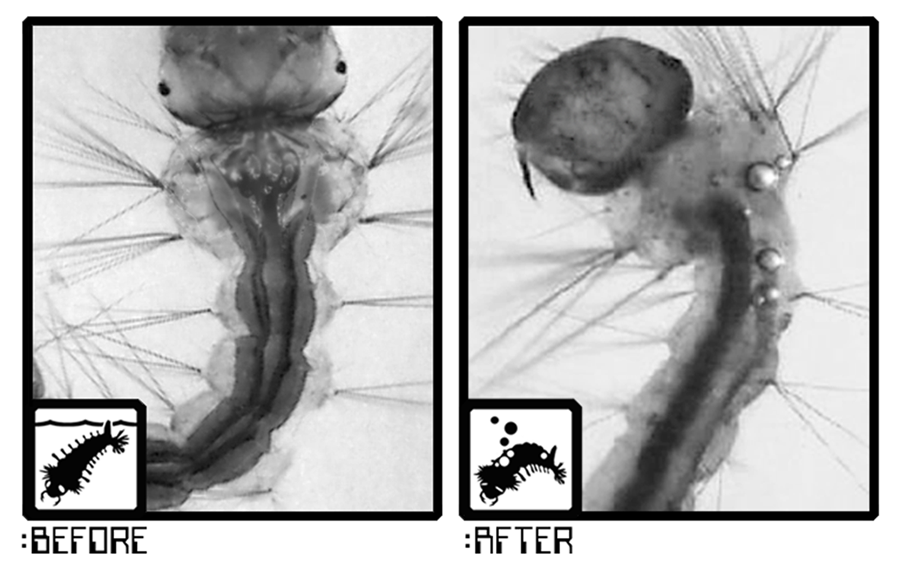
Mosquito larvae breathe through a delicate structure called the dorsal tracheal trunk, which contains tiny air bubbles. These bubbles vary in size depending on mosquito species and the stage of larval growth.
Acoustic Larvicide® works by sweeping through carefully tuned frequencies until resonance is achieved with these air bubbles. When resonance occurs, the bubble vibrates violently, rupturing the membrane of the larva’s respiratory system. This causes gas to escape upward through the body — leading to both instant mortality and delayed effects in survivors.
Power and Frequency
- Small larvae (<3.5 mm) resonate differently than
- Large larvae (>3.5 mm)
The chart below shows the transducer power vs. frequency for both groups.
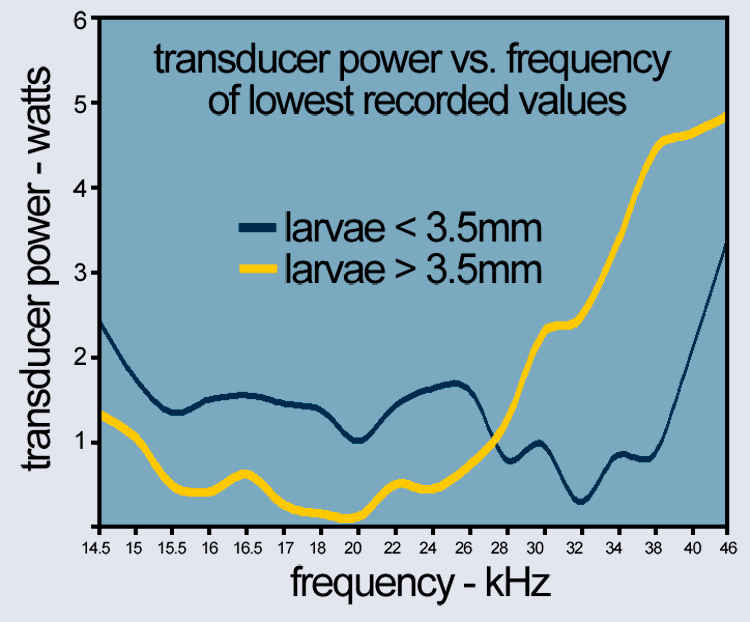
By sweeping across these ranges, Acoustic Larvicide® ensures comprehensive coverage of mosquito populations at different stages of growth.
Why Sound Works
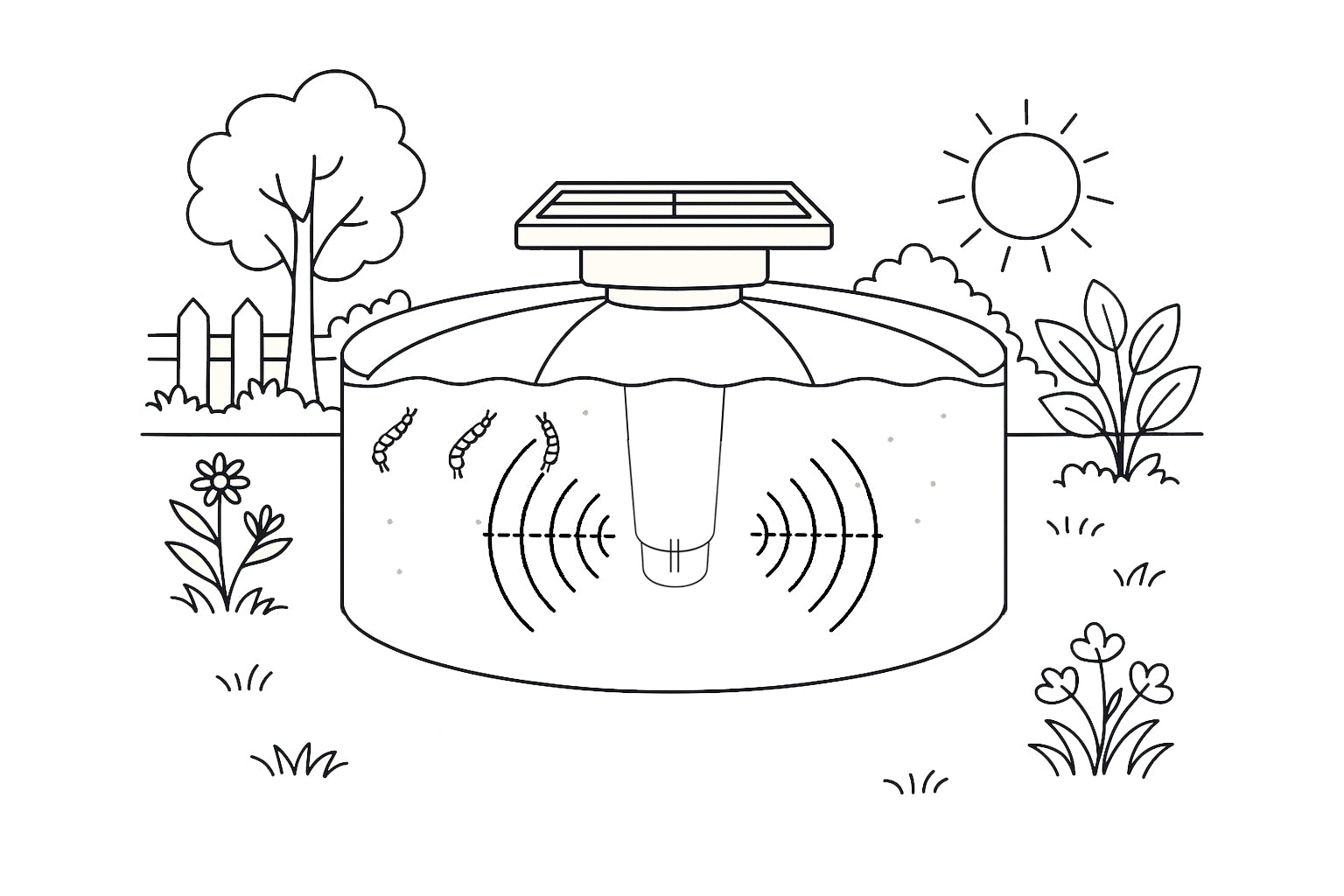
- Larvae live in water and breathe at the surface.
- Specific sound waves couple efficiently into water.
- Pressure changes and vibration disrupt larval breathing and development.
- Stopping larvae prevents the next generation before it can fly and bite.
Illustration: sound propagating inside a Sirenix device, highlighting larval breathing zones where acoustic disruption occurs.
Key Advantages of Acoustic Larviciding
- Chemical-Free: No pesticides, no toxins, no residues in water.
- Targeted: Frequencies tuned to mosquito larvae air bubbles; harmless to fish, amphibians, pets, and people.
- Sustainable: Powered by solar energy, operates automatically with minimal upkeep.
- Effective: Breaks the breeding cycle at its source, collapsing mosquito populations within weeks.
Applications
Acoustic Larvicide® can be applied in:
- Residential backyards and gardens
- Commercial landscapes
- Agricultural irrigation ponds
- Municipal stormwater systems
- Wetlands and conservation zones
Comparative Matrix
Side-by-side view of control methods across key evaluation criteria.
| Criterion | Acoustic Larviciding | Chemical Larvicide | CO₂ Trap (Adult) |
|---|---|---|---|
| Primary target | Larvae (pre-adult; breeding sites) | Larvae (pre-adult; breeding sites) | Adults (biting stage) |
| Efficacy window | Continuous, timed pulses | Days–weeks; depends on residual & dilution | Instant while powered; no breeding interruption |
| Non-target impact | Highly selective (frequency-specific resonance) | Variable; may affect aquatic microbiota/inverts | Low; may capture non-target flying insects |
| Maintenance | Add water; clean quarterly; solar-powered | Re-dose cycles; storage/handling requirements | Propane/CO₂ refills; net changes; frequent checks |
| Cost / year | Low ongoing (solar); initial device cost | Consumables + labor re-applications | Fuel/consumables + net replacements |
| Outcome on population | Breaks breeding cycle → population collapse | Breaks breeding cycle (if re-dosed on schedule) | Does not interrupt breeding; reduces biting pressure only |
Note: Costs and maintenance vary by deployment scale, water turnover, and local conditions.
Key Scientific Information
Acoustic exposure levels vs. non-target organisms
Attenuation with depth, turbidity, and algae load
Container geometry and boundary effects
Duty cycle and operational cadence
Water chemistry considerations (pH, hardness, organics)
Species and instar variability
Environmental and occupational safety
Comparison with chemical larvicides
Monitoring and verification of efficacy
Scalability and network placement
References
- Scientific Reports (2020): Acoustic tracheal rupture provides insights into larval mosquito respiration
- Journal of the American Mosquito Control Association (2016): Acoustic control of mosquito larvae in artificial drinking water containers
- Applied Sciences (2020): Ultrasonic Technology Applied against Mosquito Larvae
- Biomedical Research (2019): Evaluation of Perseus ultrasonic larvicidal device
- Journal of the Florida Mosquito Control Association (2023): Field evaluation of Autocidal Gravid Ovitrap and SIRENIX trap against container-inhabiting mosquitoes in Saint Augustine, Northeastern Florida
